Background: Acute myeloid leukemia (AML) is an aggressive hematologic malignancy carrying a poor prognosis. Relative to non-leukemic myeloid progenitors, AML cells present with elevations in mitochondrial metabolism driven by higher mitochondrial content. Despite having more mitochondria per cell, AML cells are inefficient at coupling respiration to ATP synthesis, indicative of intrinsic deficiencies in oxidative phosphorylation (OXPHOS) flux. The present study aimed to determine the relationship between mitochondrial OXPHOS kinetics and chemotherapy response in a clinical cohort of AML patients by comparing mitochondrial bioenergetic profiles between responders to induction chemotherapy with those who have persistent disease, and between adverse risk and intermediate risk AML.
Methods: In this prospective study from January 2020 to February 2023, we collected bone marrow aspirate samples from patients with new diagnosis of AML on day 1 to evaluate the bioenergetic phenotype of the AML cells. We collected data on primary induction therapy, response to induction therapy, relapse, and other clinical features. We compared the bioenergetic phenotypes between the responders to primary induction chemotherapy and non-responders who had persistent disease post induction chemotherapy. We did the same comparison between patients with adverse risk and intermediate risk AML per European LeukemiaNet (ELN) 2022 risk stratification. Mitochondrial bioenergetic profiles were generated via high resolution respirometry using freshly isolated mononuclear cells from each patient's bone marrow aspirate. The Fisher exact test was used for categorical variables and the Wilcoxon Rank Sum for continuous variables.
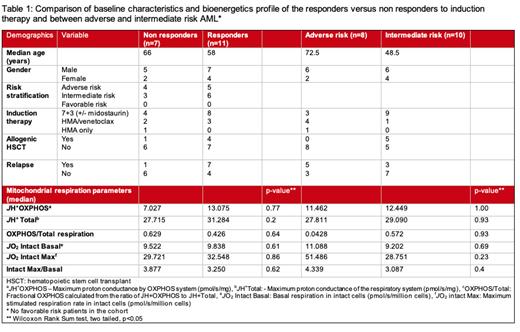
Results: In 18 AML patients included in the study, median age was 64 years (range 30-85 years), 67% (N=12) were male, 33% female, 61%(n=11) were Caucasian and 39%(n=7) were African Americans. Day 1 median blast count was 57% (range 20-92%). Initial induction therapy with cytarabine/daunorubicin (standard 7+3) with or without midostaurin (in patients with FLT3 mutations) was done in 12 (67%) patients and hypomethylating agent/venetoclax was used in 5 (28%) patients. One patient was managed with azacitidine only. Seven (39%) patients had persistent disease after induction and others responded to the initial induction. Relapsed disease after achieving remission with initial induction was seen in 8 (44%) patients. No patients had favorable risk cytogenetics/molecular findings, 10 had adverse risk AML and 9 had intermediate risk AML. TP53 mutation was observed in 4 patients, FLT3 mutation in 5 patients, IDH1 mutation in 4, and IDH2 mutation in 3 patients. The fraction of the respiratory network capable of performing OXPHOS (i.e., Fractional OXPHOS) was 0.426 in responders and 0.629 in non-responders (p=0.93). In responders, basal respiration in intact cells was non-significantly (p=0.54) lower ( JO2 9.838 pmol/s/million cells) compared to non-responders ( JO2 11.52 pmol/s/million cells). Adverse risk patients had lower fractional OXPHOS, and higher basal and maximal respiration rates as compared to intermediate risk AML patients (Table 1).
Conclusion: While Fractional OXPHOS and basal respiration were lower in the patients who had remission after induction therapy, this difference was not statistically significant. We did not find a clinically significant correlation in the bioenergetic profile of AML cells; however, our study is limited by small sample size. Further research is warranted to investigate the role of OXPHOS kinetics in patients of AML that can translate into clinically meaningful outcomes.
Disclosures
Liles:Abbvie: Other: Clinical trial activity (Principal investigator or sub-investigator); Alpine Immune Sciences: Other: Clinical trial activity (Principal investigator or sub-investigator); Annexon Biosciences: Other: Clinical trial activity (Principal investigator or sub-investigator); Astex Pharmaceuticals: Other: Clinical trial activity (Principal investigator or sub-investigator); Baxalta: Other: Clinical trial activity (Principal investigator or sub-investigator); BeiGene: Other: Clinical trial activity (Principal investigator or sub-investigator); Bioverativ: Other: Clinical trial activity (Principal investigator or sub-investigator); CSL Behring: Other: Clinical trial activity (Principal investigator or sub-investigator); Celgene: Other: Clinical trial activity (Principal investigator or sub-investigator); Delta-Fly Pharma: Other: Clinical trial activity (Principal investigator or sub-investigator); Exact Sciences: Other: Clinical trial activity (Principal investigator or sub-investigator); Forma Therapeutics: Other: Clinical trial activity (Principal investigator or sub-investigator); Global Blood Therapeutics: Other: Clinical trial activity (Principal investigator or sub-investigator); Immunovant: Other: Clinical trial activity (Principal investigator or sub-investigator); Incyte: Other: Clinical trial activity (Principal investigator or sub-investigator); Janssen Pharmaceuticals: Other: Clinical trial activity (Principal investigator or sub-investigator); NeoImmuneTech: Other: Clinical trial activity (Principal investigator or sub-investigator); Novartis: Other: Clinical trial activity (Principal investigator or sub-investigator); Novo Nordisk: Other: Clinical trial activity (Principal investigator or sub-investigator); Partner Therapeutics: Other: Clinical trial activity (Principal investigator or sub-investigator); Pharm-Olam: Other: Clinical trial activity (Principal investigator or sub-investigator); Principia Biopharma: Other: Clinical trial activity (Principal investigator or sub-investigator); Salix Pharmaceuticals: Other: Clinical trial activity (Principal investigator or sub-investigator); Sanofi-Aventis: Other: Clinical trial activity (Principal investigator or sub-investigator); Takeda: Other: Clinical trial activity (Principal investigator or sub-investigator); Vifor Pharma: Other: Clinical trial activity (Principal investigator or sub-investigator).